734
Views & Citations10
Likes & Shares
Seed is the most important part of plant for
propagation. Proper storage of seed in suitable container in viable condition
for future use is a meticulous task. Insane application of artificial seed
protectants is producing lethal effects on general public and environment.
Therefore, in present study naturally available plants as seed protectant are
tried on seed of Pigeon pea stored in four containers; gunny bag, tin box,
plastic bag, glass bottle. Plant powders of Azadirachta
indica A. Juss, Cyperus rodundus L. and Ocimum basilicum L. found useful to be as seed protectant for test
pulse.
Keywords: Seed, Propagation, Pigeon
pea (Cajanas cajan)
INTRODUCTION
Pigeon pea (Cajanas cajan L.) is an annual shrub of about 6-7 feet. The
inflorescence is a typical axillary raceme bearing papilionaceous flowers. It
is cultivated as a mixed crop with Kharif cereals in low rainfall areas. Sowing
is done in June-July and harvested after 6-8 months, between January-February.
It is commonly cultivated in Tamil Nadu, Bihar, Rajasthan, Maharashtra, Orissa
and Uttar Pradesh.
Pigeon pea contains protein 20.4 g/100 g of
seeds and carbohydrates are 60.4 g/100 g of seeds suggesting that it is also
good source of protein and carbohydrates, it also contain thiamin (0.45 mg),
niacin (2-9 mg) and riboflavin (0.19 mg). It has better quality of fiber (7
g/100 g of seeds) [1].
Storage and preservation of seed of crop is now a day is done using fungicides and pesticides of inorganic origin. Unabated application of artificial seed protectants is causing holistic damage to the ecosystem. Ideally a pesticide must be lethal to the targeted pest, but not to non-targeted beings, including human. Unfortunately, this is not the case, so the controversy of use and abuse of pesticide has surfaced. The rampant use of these chemicals caused havoc to the living forms [2,3] found effectiveness of tobacco leaf powder in controlling oviposition, adult emergence of Callosobruchus chinensis pest causing seed infestation to stored Chickpea. Rajapakse [4] successfully used some plants to control infestation of beetles on stored seed crops.
MATERIALS AND METHODS
Collection of test pulse, plants and
preparation of plant parts powder
Pigeon pea (Cajanas cajan L.) collected from local farms and market in Nanded
district of Maharashtra, India. The test treatment plants; Azadirachta indica A. Juss., Ocimum
basilicum L. and Cyperus rotundus
L.; used as bio-powder plant protectants, collected from local area of Nanded
district, Maharashtra, India and identified from their morphological characters
using ‘Flora of Marathwada’ [5]. Plants were cut, separated into different
parts stem, leaves, root, surface sterilized with 0.1% HgCl2 and
washed to remove disinfectant with sterile distilled water. Sterilized plant
parts kept for drying in hot air oven at 60°C for 48 h.
Application of plant part powders to seed of
test pulse Pigeon pea
The dried plant parts leaf, stem and root
crushed to powder with the help of grinder. The test plant powders thus
obtained passed through sieve to get fine powder and stored in polythene bags.
1 kg seed of Pigeon pea dusted separately
with ten gram leaf powder of Azadirachta
indica A. Juss, Ocimum basilicum
L. and rhizome powder of Cyperus rotundus
L. Treated seed of the pulse were stored in different containers like gunny
bag, plastic bag, tin box and glass bottle. After storing seed of test pulse in
different containers for one year, the seeds were incubated on moist blotters
for ten days at room temperature. On eleventh day seed health in terms of seed
mycoflora, seed germination, root and shoot length was studied. Seeds without
dusting with any plant part powder served as control.
RESULTS AND
DISCUSSION
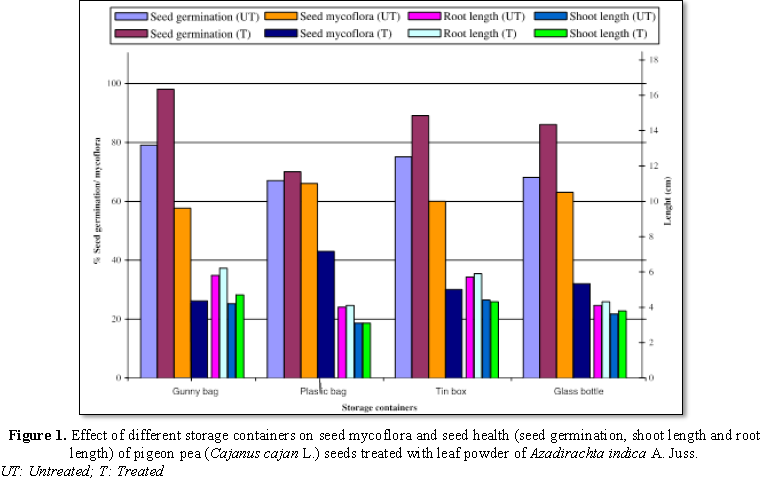
The results in the Figure 1 show, seed from all the containers; treated with leaf
powder of Azadirachta indica A. Juss.
showed reduced seed mycoflora and enhanced seed germination, shoot and root
length. As regards to untreated seeds maximum seed mycoflora was observed in
the seeds stored in plastic bag (66%), followed by glass bottle (63%) and least
in gunny bag (59%). Treated seeds stored in plastic bag showed maximum seed
mycoflora (43%) and least in gunny bag (27%).
Seed germination was reported to be increased
in treated seeds than in untreated seeds stored in all containers. Maximum seed
germination in case of treated seeds was noticed in the seeds stored in gunny
bag (98%), followed by tin box (89%) and minimum in plastic bag (70%).
Untreated seeds stored in gunny bag showed maximum seed germination (79%) and
least in plastic bag (67%). shoot and root lengths were increased in treated
seeds over untreated ones, stored in all containers. shoot and root lengths
were a bit more in untreated and treated seeds stored in gunny bag.
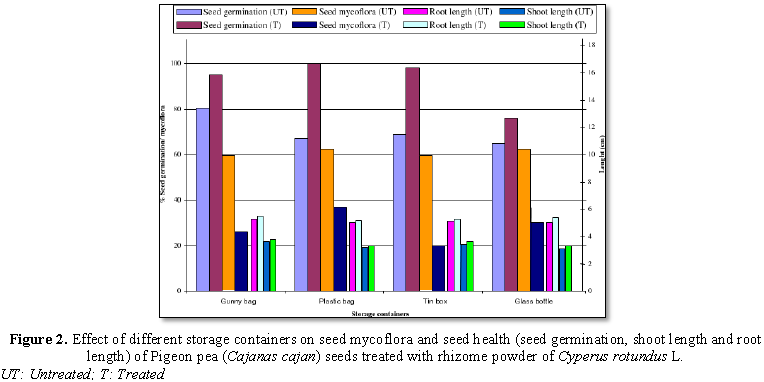
Seed treated with rhizome powder of Cyperus rotundus L. Figure 2 indicates reduced seed mycoflora and increased seed
germination, shoot and root length stored in all the containers. Untreated
seeds stored in plastic bag showed maximum seed mycoflora (62%) and least in
gunny bag and tin box (60% each). Treated seeds stored in plastic bag showed
maximum seed mycoflora (37%) and least in tin box (20%), followed by gunny bag
(26%).
Seed germination was significantly increased
in treated seeds than in untreated seeds, stored in all containers. Maximum
seed germination in untreated seeds was recorded in gunny bag (80%), followed
by tin box (69%) and least in glass bottle (65%). Similarly maximum seed
germination in treated seeds was recorded in the seeds stored in plastic bag
(100%), followed by tin box (98%) and minimum in glass bottle (76%). Shoot and
root lengths were found to be increased in treated seeds than in untreated
seeds, stored in all containers. Shoot and root lengths in treated seeds showed
more or less increase in the seeds stored in all containers.
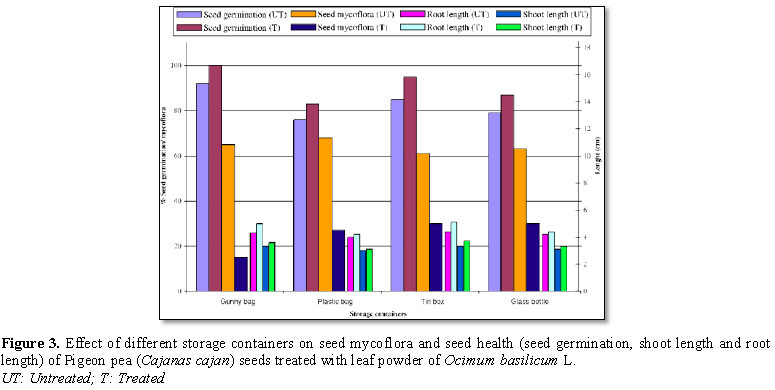
Figure 3 show seeds treated with Ocimum basilicum L. showed reduced seed
mycoflora and increased seed germination, shoot and root length in all the
containers. Seed mycoflora of treated seeds was found to be reduced compared to
untreated seeds stored in all containers. In case of untreated seeds, maximum
seed mycoflora was recorded in the seeds stored in plastic bag (68%) and
minimum in tin box (61%); followed by glass bottle (63%). Treated seeds stored
in tin box and glass bottle showed maximum seed mycoflora (30% each), whereas
least was recorded in gunny bag (15%), followed by plastic bag (27%).
Seed germination was considerably improved in
treated seeds over untreated seeds, stored in all containers. Maximum seed
germination, in case of untreated seeds was noticed in the seeds stored in
gunny bag (92%) and minimum in plastic bag (76%). Treated seeds stored in gunny
showed maximum seed germination (100%), followed by the seeds stored in tin box
(95%) and least in plastic bag (83%). Shoot and root lengths were increased in
treated seeds than in untreated ones, stored in all containers. Treated seeds
showed increased shoot and root lengths in all the containers in more or less
quantity.
Similarly, Naik [6], found difference in
fungal flora under different storage periods, four months stored seeds nurtured
Chaetomium globosum, C. spirata, Rhizopus arrhizus and Penicillium
spp. and eight month stored seeds developed mainly Aspergillus fumigatus, A.
sydowii, A. flavus and A. niger. Singh and Singh [7] studied
seed mycoflora of mustard, linseed, sunflower, safflower, soybean, sesame and
groundnut recorded that, the fungi like Alternaria, Cladosporium, Curvularia,
Fusarium and Helminthosporium decreased gradually during storage period and
disappeared after three years and were succeeded by storage fungi like Aspergillus spp., Penicillium spp. and Rhizopus
spp. Bhattacharya and Radha [8] recorded fungal infection, moisture content,
germinability and deterioration of seeds of maize, groundnut and soybean in
storage at the locality of Santiniketan, West Bengal, India under natural
condition for one year. Dominant fungi recorded from stored seeds were Aspergillus candidus, A. flavus, A. niger, A. terreus, A. ruber, Rhizopus spp. Penicillium
spp., Curvularia spp., Fusarium spp. Alternaria spp., etc. Carbohydrates and protein content of the test
seeds were found to be declined. Jurjevic et al. [9] studied changes in fungi
and mycotoxins in pearl millet under controlled storage conditions; further
they reported that, predominant fungi showed fluctuation in their incidence
with changes in storage conditions such as temperature, moisture and humidity.
Abdulaziz et al. [10] reported that storage of Ephedra alta seeds in cotton cloth bags favorably maintained seed
moisture content below critical level resulting in minimum seed deterioration
compared with other seed storage containers. Khatun et al. [11] used
botanicals, such as whole leaf powder of Neem (Azadirachta indica), Ipomoea
sepiara and Polygonum hydropiper
at a dose of 5% w/w (25 g botanical per 500 g of lentil seeds). In addition, Polygonum hydropiper L. were effective
in preserving seed germination and seed vigor of lentil. Gopinath et al. [12]
found that storage fungi reduced total fat (1.94-1.75 g), triglycerides
(1.46-1.07 g), whereas phospholipids (0.06-0.21 g), free fatty acids
(0.002-0.01 g) and peroxide values increased. The fatty acid content of
palmitic, steric, linoleic acid decreased, but oleic acid content increased in
red gram and chickpea during storage periods. Khalequzzaman et al. [13] found
moisture content, seed weight, abnormal seedlings, seed rot and fungal
association of French bean increased, but germination and normal seedlings
growth decreased with increase in storage period. Kakade and Chavan [14]
reported negative nutritional and fatty oil alteration in soybean and safflower
due to storage fungi; like Alternaria
sp., Fusarium sp., Macrophomina sp., Curvularia sp., Rhizopus
sp., Penicillium sp., etc.
Sethumadhava et al. [15] reported that storage fungi like Aspergillus flavus, A. niger,
A. fumigatus, Cladosporium cladosporiodes, etc., reduced carbohydrates, amino
acids and phenols in the vegetables, increased storage period abnormally
increased phenols and amount of reducing sugar. Dubale et al. [16] screened
seed mycoflora of maize (Zea mays L.)
stored in traditional storage container Gombisa and sacks, common fungi
reported were Aspergillus flavus, A. fumigatus, A. niger, A. terreus, Cladosporium cladosporiodes, Drechslera halodes, Fusarium oxysporum and Penicillium
chrysogenum. Lambat et al. [17] reported polyethylene bag imparted much
protection. Nazareth et al. [18] found Neem and Tulsi powder individually and
synergistically (1:1) anti-fungicidal against mycoflora of four oil seeds; it
also enhanced germination, emergence, shoot and root length of oil seeds.
In vitro application of the selected
plants could be used on larger scale in storage houses to eschew artificial
harmful preservatives. It could be better replacement for the chemical plant
protectants to protect the post-harvest products. The environmental degradation
could be retarded to some extent as earth and its biome is at serious threshold
of deterioration. All countries must heed the warnings of the ‘union of
concerned scientists’ to protect the earth for future generations [19].
1. Shakuntala Manay N,
Shadaksharaswamy M (1987) Foods: Facts and principals. Wiley Eastern Limited.
2. Aktar MW, Sengupta D, Chowdhary A
(2009) Impact of pesticides use in agriculture: Their benefits and hazards.
Interdiscip Toxicol 2: 1-12.
3. Hossaini MA, Bachchu MAA, Ahmed
KS, Haque (2014) Effectiveness of indigenous plant powders as grain protectant
against Callosobruchus chinensis L.
in stored chickpea (Cicer arietinum
L.). Bangladesh J Agric Res 39: 93-103.
4. Rajapakse RHS (2006) The potential
of plant products in stored insect pest management. J Agric Sci 2.
5. Naik VN (1998) Flora of
Marathwada. Vol. I and II, by Amrut prakashan, Aurangabad, India, p: 1182.
6. Singh BK, Singh S (1979)
Prevalence of fungi and their role on activities of the seeds of three oil
yielding crop. Seeds Farms 5: 27-29.
7. Chadra S, Narang M, Shrivastava RK
(1981) Changes in association of mycoflora and viability of seeds in some oil
seeds in prolonged storage. Geobios 8: 200-204.
8. Bhattacharya K, Radha S (2002)
Deteriorative changes of maize, groundnut and soybean seeds by fungi in
storage. Mycopathologia 155: 135-141.
9. Jurjevic Z, Wilson J, Wilson D,
Casper H (2007) Changes in fungi and mycotoxins in Pearl millet under
controlled storage condition. Mycopathologia, pp: 146-159.
10. Abdulaziz A, Al-Qarawi Ilsayed F,
Allah A (2010) Maintenance of Ephedra
alta seeds viability via storage containers. Am J Plant Sci 1: 138-146.
11. Khatun AG, Kabir MA, Bhuiyan H,
Khanam D (2011) Effect of preserved seeds using different botanicals on seed
quality of lentil. Bangladesh J Agric Res 36: 381-387.
12. Gopinath R, Sambiah K, Niranjana
SR (2011) Effect of storage on red gram (Cajanas
cajan L. Mill sp.) and green gram (Vigna
radiata L. Wilczek) with particular reference to lipid composition Plant
Protect Sci 47: 157-165.
13. Khalequzzaman KM, Rashid MM, Hasan
MA, Reza MMA (2012) Effect of storage containers and storage periods on the
seed quality of French bean (Phaseolus
vulgaris). Bangladesh J Agric Res 37:
195-205.
14. Kakade RB, Chavan AM (2012)
Nutritional changes in soybean and safflower oil due to storage fungi. Curr Bot
3: 18-23.
15. Rao S, Laxmi Narayana GS,
Bhadraiah B, Manoharachary C (2014) Biochemical changes due to fungal
infestation in stored seeds of some vegetable crops. Indian Phytopathol 67:
159-163.
16. Dubale B, Solomon A, Geremew B,
Sethumadhava Rao G, Waktole S (2014) Mycoflora of grain maize (Zea mays L.) stored in traditional
storage containers (Gombisa and Sacks) in selected areas of Jimma zone,
Ethiopia. Afr J Food Agric Nutr Dev 14: 2.
17. Lambat A, Lambat P, Gdewar R,
Charjan S, Charde PN (2015) Effect of storage containers on mycoflora and
germinability of Til. Int J Res Biosci Agric Technol 3: 10-12.
18. Nazareth MS, Girish K, Fathima SK
(2018) Efficacy of herbal powders on seed mycoflora and seed quality of
oilseeds. J Biopesticides 11: 106-113.
19. Ripple WJ, Wolf C, Newsome TM,
Galetti M, Alamgir M, et al. (2017) Laurance and 15,364 scientist signatories
from 184 countries. World scientists’ warning to humanity: A second notice.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Astronomy and Space Research
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)